Accurate and Affordable Quantum Chemistry with the Kvantify Chemistry QDK on IQM Resonance Hardware

Published on June 23, 2025
Patrick Ettenhuber, pier P. Poier
In this post we showcase how the Kvantify Chemistry Quantum Development Kit (QDK) has allowed us to replicate a 2023 study of butyronitrile by IBM Quantum on IQM Resonance hardware using the 16- and 20-qubit Sirius and Garnet systems. On quantum systems this big, classical processing can become a limiting factor, and the Kvantify Chemistry QDK is unique in being able to perform accurate real-world chemistry calculations that fully utilize large devices without excessive classical compute and with an affordable number of quantum-hardware operations.
With few exceptions for academic demonstrations, quantum software for chemical applications cannot make use of the most performant quantum hardware that is commercially available today, such as the 16- and 20-qubit devices available in the IQM Resonance Cloud. Limitations are many, and in particular hardware noise often requires a substantial part of the quantum-amenable calculation to be simulated with a noise-free classical simulator. In the regime of 20+ qubits, exact quantum simulation becomes a bottleneck, and the Kvantify Chemistry QDK is the successful result of our efforts to develop and implement algorithms that make full use of the quantum hardware to allow scalable quantum-chemistry calculations on ever larger hardware.
To demonstrate the capabilities of the QDK, we use it to simulate the dissociation of the nitrile group in butyronitrile, a process which is relevant for a number of technical applications as detailed in a later section. This study was originally carried out on quantum hardware by IBM in 2023 using a minimal basis set and the well-known ADAPT-VQE algorithm on eight hardware qubits.

Visualization of electron densities and chemical bounds during the nitrogen dissociation process: The moving atom is the nitrogen atom, carbon atoms are shown in dark gray, and hydrogen atoms in white. (Lewis) electron pairs are indicated as black connections between the atoms, and under the bond-breaking process, the (triple) bond between nitrogen and carbon is broken. A more realistic picture of the chemical bonds is shown by the light-green and dark-green electron densities derived from quantum-chemical calculations. The light-green part of the electron density is the part for which an effective Hamiltonian is constructed for the calculations on quantum hardware, and the dark-green part is the (static) environment that enters into the effective Hamiltonian.
To model the dissociation, we compute the system energy at discrete points in the process (corresponding to frames in the figure above). This was a tremendous effort in 2023, but with the Kvantify Chemistry QDK we can effortlessly replicate the study using a manageable budget while obtaining accurate results.
In the original work by IBM Quantum, only the final energy evaluation was carried out on a quantum computer, while the calculations required to establish the wave-function representation were performed on a quantum simulator running on a classical computer. The Kvantify Chemistry QDK instead relies on Kvantify’s patented FAST-VQE solution to run the adaptive operator selection step using quantum hardware (here IQM Garnet or IQM Sirius) for circuit sampling, while the VQE part is simulated on Kvantify’s state-vector simulator, which is optimized for chemistry. This approach uses quantum hardware for operations with low shot-counts and high error resilience, for which it is well suited, while avoiding the high-shot-count requirements for energy evaluation imposed by e.g. ADAPT-VQE.

Visualization of the iteration process of Kvantify’s patented FAST-VQE method discussed above.
To run the calculation on quantum hardware, we run 60 iterations of the adaptive algorithm, for each separation distance. Each iteration uses 1024 shots for circuit sampling, corresponding to 2-3 seconds of quantum runtime. Based on this, we can give the following estimate of on-demand (non-discounted list) prices to run a single point or the full calculation on various hardware platforms:
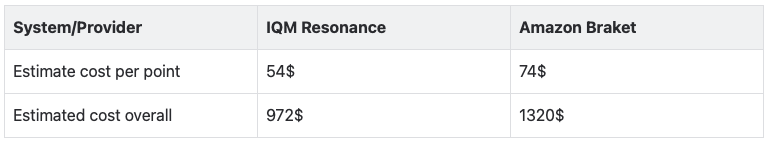
The Kvantify Chemistry QDK integrates directly with the IQM Resonance Cloud, as well as other providers, and provides an economically and technologically accessible route to running quantum experiments for chemistry calculations on hybrid quantum-classical systems. The QDK aims to make quantum chemistry calculations on quantum hardware accessible to computational chemists without requiring them to become experts on quantum algorithms or technology and will soon be available for general use – get in touch to learn more.
Results
Below we show the raw results of computing the dissociation curves (energy as function of nitrogen distance) using IQM hardware and the Kvantify Chemistry QDK for an increasing number of spin orbitals, corresponding to an increasing number of utilized hardware qubits.
In addition to the benefit of larger active spaces (i.e. qubit counts), the calculations benefit from a couple of enhancements compared to the original study, including:
- using a realistic basis set PCSEG-2 instead of STO-3G;
- using the even-handed subsystem selection to ensure a consistent selection of orbitals for projective-embedding calculations in chemical reactions.
The plots below show the results of our calculations as potential energy surface (PES), i.e. the energy of the whole chemical system at various distances between the nitrogen and carbon atom, as computed via the FAST algorithm as shown above and using either IQM's Sirius or Garnet devices and by Kvantify’s exact chemistry-specific state-vector simulator. Both results are compared to the exact results obtained in the same orbital space (CASCI), the difference being plotted in the lower parts of the figures.
The experiments are conducted for an increasing number of spin orbitals, eventually exhausting each of the quantum chips. We can see clearly how the errors, irrespective of the device or simulator, follow each other, with the highest errors obtained in the dissociation limit. This is expected, since here, the Hartree-Fock state is expected to be furthest from the actual state, and thus more adaptive iterations are required to obtain the same accuracy, but in general the FAST curves are smooth and follow the CASCI curve closely on both machines. With 16 qubits, which exhausts the IQM Sirius machine, the calculation has not fully converged after 60 iterations, but convergence is still smooth towards the CASCI results. The same effect can be seen even stronger with 20 spin orbitals, exhausting the IQM Garnet chip.

Dissociation curve computed with 12 spin orbitals on IQM Garnet and Sirius hardware

Dissociation curves computed with 16 spin orbitals on IQM Sirius hardware

Dissociation curves computed with 20 spin orbitals on IQM Garnet hardware
The case for butyronitrile
Butyronitrile is an interesting subject of study for both scientific research and technological applications.
From a broader perspective, butyronitrile has emerged in recent years as a promising electrolyte candidate in advanced technologies such as lithium-ion batteries and dye-sensitized solar cells (DSSCs). These applications are key components of ongoing efforts toward a sustainable energy transition, which, while environmentally essential, also introduce significant technical challenges.
DSSCs are regarded as a viable and cost-effective alternative to conventional p–n junction photovoltaic devices. Their commercial deployment is anticipated to become competitive with fossil fuel-based energy production within the next decade. Among the advantages of DSSCs are their favorable performance-to-cost ratio and their long operational lifetimes, attributed to minimal degradation under solar exposure. This makes them particularly attractive from both economic and environmental perspectives. However, improving their power conversion efficiency remains a critical hurdle. This primarily depends on the development of optimized electrolytes that can maintain desirable properties across both high and low temperature ranges.
Currently, liquid electrolytes perform adequately at elevated temperatures but suffer from high viscosity and freezing issues at lower temperatures, which limit their usability. Conversely, more volatile electrolytes are prone to evaporation or degradation above approximately 60°C. Therefore, the development of high-performance electrolytes that remain functional across a wide temperature spectrum is a primary focus in DSSC research.
Similar electrolyte-related limitations are encountered in lithium-ion batteries, especially under low-temperature conditions where increased viscosity reduces ion mobility between electrodes, thereby diminishing battery performance. Another critical issue arises at high voltages, where electrolyte decomposition at the cathode surface leads to efficiency loss and reduced battery lifespan.
In both contexts, butyronitrile has shown significant promise due to its favorable physico-chemical properties, including low viscosity at reduced temperatures and chemical stability against cathode oxidation. These characteristics make it a strong candidate for improving the reliability and performance of next-generation energy storage and conversion technologies.